ProKidney
Founded in 2015, PROK is developing a novel therapy to improve or reverse decline in kidney function without the need for immune suppressive agents. The company’s lead product candidate, REACT, for which it has royalty-free economics, is a patented disease-modifying autologous cell therapy produced from a patient’s own kidney cells. Chronic kidney disease (CKD) is one of the most prevalent and expensive medical conditions to treat, affecting more than 1 in 7 adults in the U.S. alone.
Currently, PROK expects to be able to fund operations to the end of 2024 with current resources. There are no other late stage cell therapies for CKD in development. While the current standard of care (SOC) for diabetic hypertensive and hypertensive CKD can slow the progress of disease, there are no approved treatments that halt or reverse progressive end-stage renal failure (ESRF). ESRF is very expensive and carries massive quality of life challenges for patients. Dialysis costs Medicare about $100,000/yr per patient. 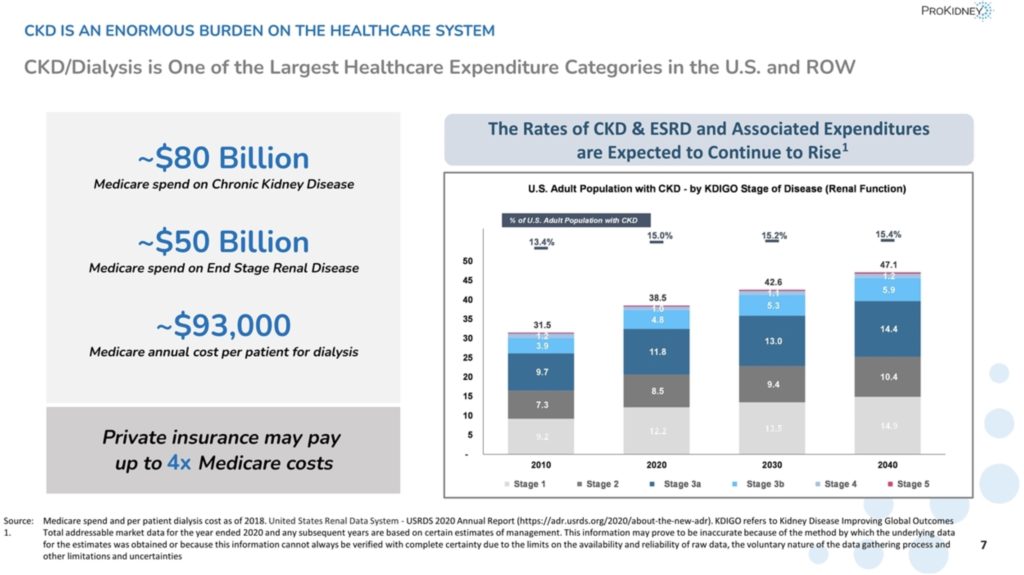 ~75M patients are diagnosed with CKD in the U.S. and EU, involving 1 in 7 (~15%) of U.S. adults. Between 2020 and 2040, the number of CKD patients is projected to grow by 22% in the U.S. and EU, in part due to the increasing prevalence of diabetes, obesity, and heart disease. According to PROK, if REACT can penetrate 1% of the addressable market and sell for $360,000 a patient, the product could bring in $16 billion.
~75M patients are diagnosed with CKD in the U.S. and EU, involving 1 in 7 (~15%) of U.S. adults. Between 2020 and 2040, the number of CKD patients is projected to grow by 22% in the U.S. and EU, in part due to the increasing prevalence of diabetes, obesity, and heart disease. According to PROK, if REACT can penetrate 1% of the addressable market and sell for $360,000 a patient, the product could bring in $16 billion.
PROK is led by a management team with 200+ years of combined experience making medicines and is chaired by Pablo Legorreta, founder and CEO of Royalty Pharma (RPRX). Chamath Palihapitiya lead the investment in PROK’s 2022 SPAC with a $125 million. Net proceeds of the July 2022 SPAC & PIPE was approximately $575 million. All directors and officers as a group own 44.6% of the company.
After birth, progenitor cells with nephrogenic potential (creation of functional nephrons) disappear, rendering adult kidneys that are unable to form new nephrons. However, there are cells within the adult kidney – tubular epithelial cells – that drive repair/regeneration of nephrons cells following acute injury, which supports PROK’s approach with REACT given that REACT cells are derived directly from the kidney through biopsy, according to a report by Bank of America Securities. By comparison, mesenchymal stem cell (MSC) therapy, which have failed to progress to late stage clinical development for the treatment of CKD, are derived from tissues outside of the kidney (bone marrow, adipocytes [fat cells]). The key development hurdle for PROK was isolating progenitor cells that could be expanded ex-vivo and then grafted within renal tissue in the patient.
PROK has presented supportive interim data from their REACT Ph2 trial (n=51), an open label trial that provided early proof-of-concept in diabetic CKD patients. REACT could be a transformative therapeutic if ongoing readouts from the Ph2 (more patients, longer follow up), and Ph3 were to replicate initial interim efficacy data.
Here are some interim data from Ph2 clinical trial in diabetic patients with CKD stages 3a, 3b, and 4 (moderate-to-severe kidney disease):
- >50% of patients with improved renal function based on eGFR
- >80% of patients with improved function are projected to never develop ESRD or need to seek renal replacement therapy
- >55% experienced normalization of anemia
These outcomes are in sharp contrast to SOC patients in the Ph2 study, where more than two-thirds were expected to progress to ESRD and dialysis.
Ph3 started enrolling this year and is aiming for up to 600 patients. Ph3 data is due in 2025. Ph3 will use a composite endpoint of time to the earliest of a 40% fall in eGFR, chronic dialysis, a 30% increase in urine albumin-creatinine ratio, or renal or cardiovascular death.
REACT has received regenerative medicine advanced therapy (RMAT) designation, allowing the potential for expedited development.
Near-term catalysts include Ph2 initial trial data looking at bilateral dosing (mid-23) and Ph2 one kidney dosing durability update (mid-23).
Updates published on SumZero:
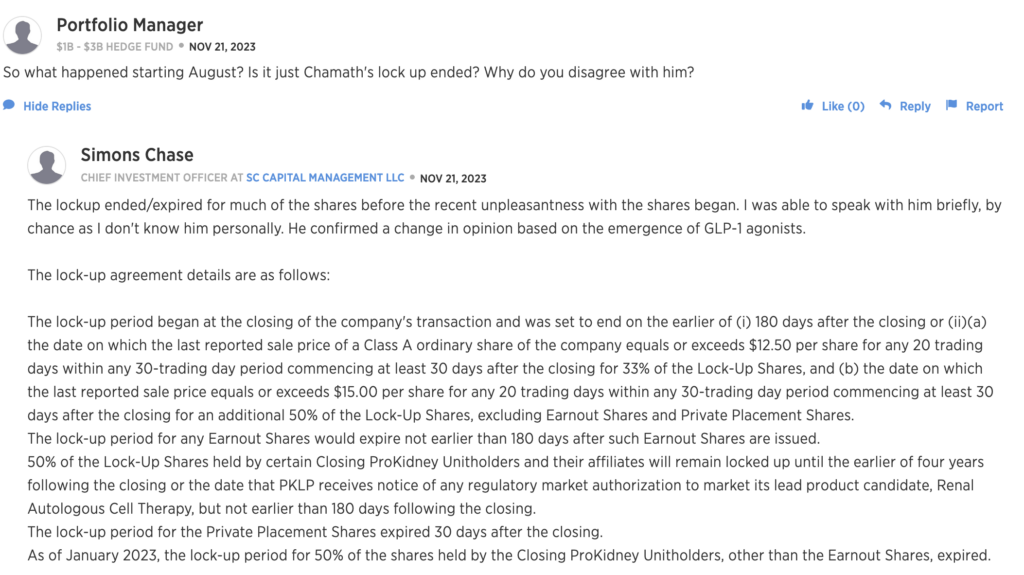
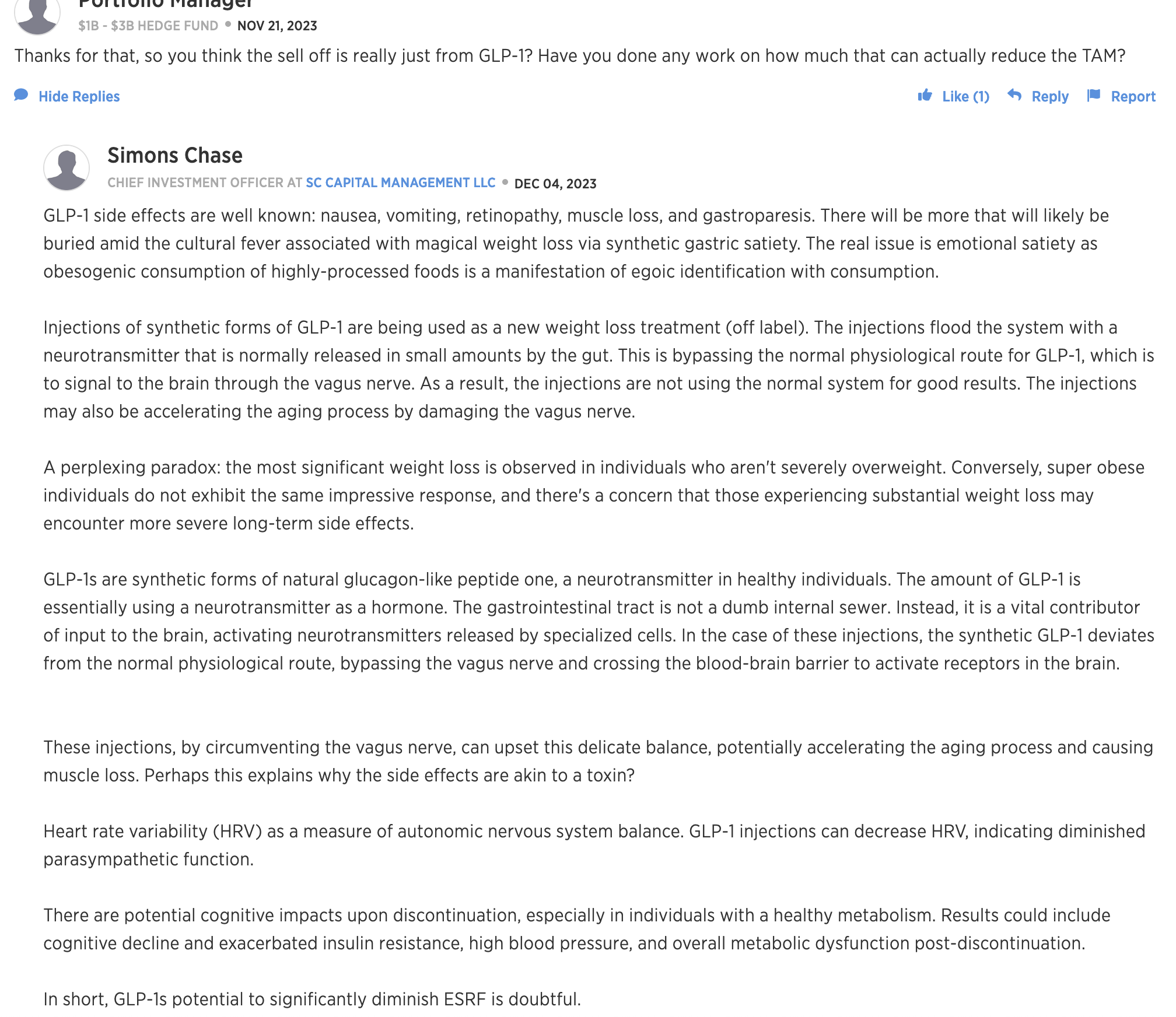
xxxx

xxxx
Investors are advised to conduct their own independent research into individual stocks before making a purchase decision. In addition, investors are advised that past stock performance is not indicative of future price action.
You should be aware of the risks involved in stock investing, and you use the material contained herein at your own risk. Neither SIMONSCHASE.CO nor any of its contributors are responsible for any errors or omissions which may have occurred. The analysis, ratings, and/or recommendations made on this site do not provide, imply, or otherwise constitute a guarantee of performance.
SIMONSCHASE.CO posts may contain financial reports and economic analysis that embody a unique view of trends and opportunities. Accuracy and completeness cannot be guaranteed. Investors should be aware of the risks involved in stock investments and the possibility of financial loss. It should not be assumed that future results will be profitable or will equal past performance, real, indicated or implied.
The material on this website are provided for information purpose only. SIMONSCHASE.CO does not accept liability for your use of the website. The website is provided on an “as is” and “as available” basis, without any representations, warranties or conditions of any kind.
Leave a Reply