Checkpoint Therapeutics Inc
Checkpoint Therapeutics Inc (CKPT) is on the cusp of becoming a significant player in the rapidly expanding immunotherapy market. Its lead candidate, cosibelimab, is a potential best-in-class anti-PD-L1 antibody, with very good clinical data through phase 3 and a PDUFA date of January 3, 2024. However, the balance sheet is a problem, in particular warrants owned by Armistice Capital.
I met with the team on December 6, 2023, and the CFO clarified the warrants as follows: $68 million assuming all warrants are exercised. There approximately 29 million warrants outstanding with an average exercise price of $2.32. Some are at $3.35, some around $2.80, and some at $1.51. The flip side of the $68 million in cash (sufficient to fund drug launch to break even) is dilution of approximately 50%.
Cosibelimab, a fully-human monoclonal antibody that targets PD-L1, is being developed for the treatment of solid tumor oncology indications, including metastatic and locally advanced cutaneous squamous cell carcinoma (CSCC). Phase 3 trials are complete and approval for marketing and sale is expected in January 2024, around the same time as the JP Morgan Healthcare Conference in San Francisco.
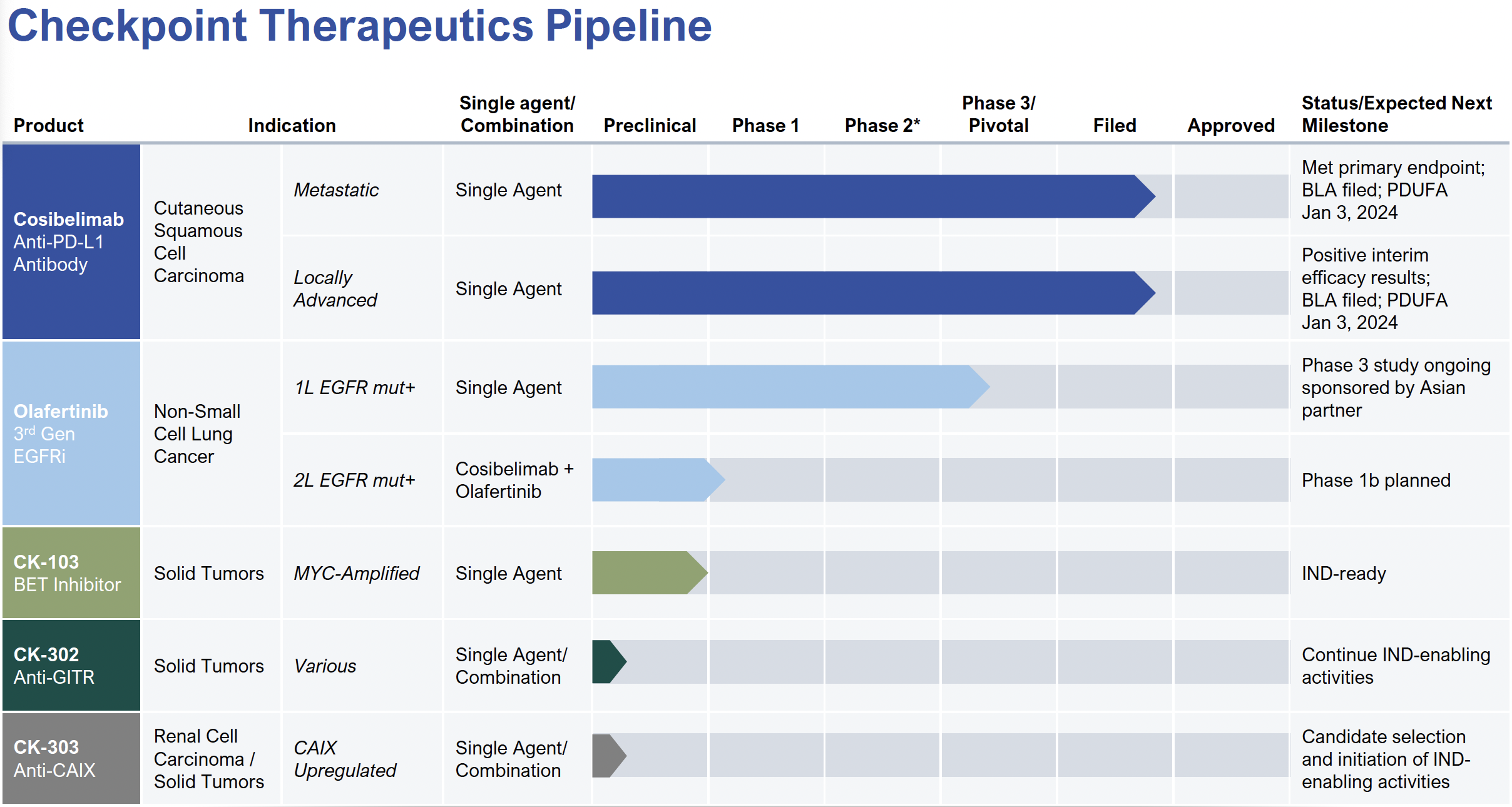
CKPT commenced a Phase 1 clinical study for cosibelimab in October 2017, evaluating its safety and tolerability in patients with selected recurrent or metastatic cancers. Following the completion of dose escalation, dose expansion cohorts were initiated, including cohorts in locally advanced and metastatic CSCC intended to support applications for marketing approval.
The company announced top-line results from a cohort of this study with cosibelimab administered as a fixed dose of 800 mg every two weeks in patients with metastatic CSCC, which met its primary endpoint with a confirmed objective response rate (ORR) of 47.4% based on independent central review. Interim results from another cohort with cosibelimab in patients with locally advanced CSCC demonstrated a confirmed ORR of 54.8%.
Based on these results, CKPT submitted a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) for cosibelimab in January 2023. The application is filed and under review, with a Prescription Drug User Fee Act (PDUFA) goal date of January 3, 2024. The company also plans to submit a marketing authorization application (MAA) in Europe in the second half of 2023, followed by additional potential submissions in markets worldwide.
Investors should note: CKPT announced longer-term results for cosibelimab from its pivotal studies in locally advanced CSCC. The results demonstrated a COMPLETE RESPOSE RATE of 55% in the locally advanced CSCC cohort based on independent central review (January 2023). These results indicate a deepening of response over time, with the median duration of response not yet reached, suggesting that responses continue to remain durable.
Here are some other key factors:
1. Addressing a Large and Growing Market: The global market for PD-L1 inhibitors is expected to reach $32 billion by 2028, driven by the rising prevalence of cancer and increasing adoption of immunotherapy.
2. Competitive Advantages of Cosibelimab: Clinical data suggests cosibelimab may offer superior efficacy and safety compared to existing PD-L1 inhibitors for CSSC (ie. Keytruda & Libtayo), potentially leading to better patient outcomes. Its unique mechanism of action targeting both PD-L1 and TIM-3 pathways could broaden its therapeutic scope and address resistance issues. Management believes its first patients will be prescribed cosibelimab as an alternative to chemotherapy as those patients make up the majority in terms of treatment of CSCC.
3. Robust Pipeline and Strategic Partnerships: Checkpoint’s pipeline includes several other promising assets in various stages of development, diversifying its portfolio and minimizing risk.
4. Cash and inventory: As of September 30, 2023, CKPT reported cash and cash equivalents of $1.7 million, or about 3-4 months of runway. This is the weakest part of CKPT’s story. It is vital for the company to obtain approval in January as this event will open up many options for liquidity. In 2023, the company purchased approximately $10 million of inventory of cosibelimab, which is secured in long-term frozen storage.
CKPT has issued several warrants with different exercise prices and expiration dates throughout 2023. Here are the details of the warrants issued:
- February 2023 Registered Direct Offering: Series A and B warrants were issued to purchase up to 1,428,572 shares of common stock each, with an exercise price of $5.00 per share. Series A warrants will expire five years after issuance, and Series B warrants will expire eighteen months after issuance.
- April 2023 Registered Direct Offering: Series A and B warrants were issued to purchase up to 1,700,000 shares of common stock each, with an exercise price of $3.35 per share. Series A warrants will expire five years after issuance, and Series B warrants will expire eighteen months after issuance .
- May 2023 Registered Direct Offering: Series A and B warrants were issued to purchase up to 3,256,269 shares of common stock each, with an exercise price of $2.821 per share. Series A warrants will expire five years after issuance, and Series B warrants will expire eighteen months after issuance.
- July 2023 Registered Direct Offering: Series A and B warrants were issued to purchase up to 3,236,248 shares of common stock each, with an exercise price of $2.84 per share. Series A warrants will expire five years after issuance, and Series B warrants will expire eighteen months after issuance.
Additionally, in October 2023, CKPT entered into an inducement offer letter agreement with a holder of certain existing warrants to exercise for cash an aggregate of 6,325,354 shares of common stock at a reduced exercise price of $1.76 per share. These warrants were originally issued with exercise prices of $4.075 and $5.00 per share. As part of this inducement, new Series A and B warrants were issued to purchase up to 6,325,354 shares of common stock each, with an exercise price of $1.51 per share. The Series A warrants will expire five years following the issuance date, and the Series B warrants will expire twenty-four months following the issuance date.
Investors should note: If the stock rises above $5, it is likely that warrant exercises will be sufficient fund the $50 million the company estimates it will need to market cosibelimab. There are other options for financing including royalties and partnerships.
Patents: The company’s U.S. patent portfolio, expiring no earlier than May 2038, provides the potential for cosibelimab to be further developed into a market leading drug, not only in CSCC, but also in additional indications, both as a monotherapy and as the PD-L1 backbone for new combination regimens.
- CAIX Antibodies: The portfolio includes three granted U.S. patents (Nos. 8,466,263, 10,450,383, and 11,174,323) related to CAIX antibodies and methods of treating cancer, with expiration dates ranging from 2027 to 2029. European and Canadian counterpart patents are also in force.
- GITR Antibodies: The GITR segment includes one granted U.S. patent (No. 10,463,732) expiring in 2035, and other national stage applications pending in various jurisdictions, with potential expiration dates no earlier than 2037.
- PD-L1 Antibodies: The PD-L1 segment includes two granted U.S. patents (Nos. 9,828,434 and 10,604,581) expiring in 2033, with additional international counterpart patents and applications pending.
- EGFR Inhibitors: The patent estate includes several granted U.S. and international patents related to small molecule inhibitors of EGFR, including olafertinib, with expiration dates in 2034 and potential patent term restorations.
- BET Inhibitors: The portfolio includes patents and applications related to small molecule inhibitors of BET, specifically targeting BRD4, with expected expiration dates in 2036.
- Licensing Agreements: CKPT has entered into licensing agreements with Dana-Farber for a portfolio of fully human immuno-oncology targeted antibodies, including antibodies targeting PD-L1, GITR, and CAIX. The agreement includes milestone payments and royalties based on net sales.
- On December 5th, the USPTO issued a new patent (U.S. Patent No. 11,834,505) covering a method of treating various cancers, including cutaneous squamous cell carcinoma (“cSCC”), through the administration of cosibelimab. The USPTO previously issued a composition of matter patent (U.S. Patent No. 10,590,199), specifically covering cosibelimab, or a fragment thereof. Together, these patents protect Checkpoint’s differentiated and potential best-in-class anti-PD-L1 antibody, cosibelimab, in the U.S. through at least May 2038, not including any potential patent term extension under the Hatch-Waxman Act.
CKPT has several royalty arrangements in place:
- With TG Therapeutics, Inc. (TGTX), CKPT is eligible to receive up to an aggregate of $60.0 million upon TGTX’s successful achievement of certain sales milestones, in addition to royalty payments based on a tiered low double-digit percentage of net sales.
- Under a collaboration agreement with Adimab, LLC, CKPT is eligible to receive payments up to an aggregate of approximately $4.8 million upon various filings for regulatory approvals to commercialize the product, as well as royalty payments based on a tiered low single-digit percentage of net sales.
- CKPT has a license agreement with Dana-Farber Cancer Institute, Inc., under which they are eligible to receive up to an aggregate of $60.0 million upon successful achievement of certain sales milestones, in addition to royalty payments based on a tiered low to mid-single digit percentage of net sales.
- A license agreement with NeuPharma, Inc. allows CKPT to receive payments of up to an aggregate of $40.0 million upon successful achievement of certain sales milestones, in addition to royalty payments based on a tiered mid to high-single digit percentage of net sales.
- CKPT also has a license agreement with Jubilant Biosys Limited, which makes them eligible to receive payments up to an aggregate of approximately $89.3 million upon successful achievement of certain sales milestones, in addition to royalty payments based on a tiered low to mid-single digit percentage of net sales
Investors are advised to conduct their own independent research into individual stocks before making a purchase decision. In addition, investors are advised that past stock performance is not indicative of future price action.
You should be aware of the risks involved in stock investing, and you use the material contained herein at your own risk. Neither SIMONSCHASE.CO nor any of its contributors are responsible for any errors or omissions which may have occurred. The analysis, ratings, and/or recommendations made on this site do not provide, imply, or otherwise constitute a guarantee of performance.
SIMONSCHASE.CO posts may contain financial reports and economic analysis that embody a unique view of trends and opportunities. Accuracy and completeness cannot be guaranteed. Investors should be aware of the risks involved in stock investments and the possibility of financial loss. It should not be assumed that future results will be profitable or will equal past performance, real, indicated or implied.
The material on this website are provided for information purpose only. SIMONSCHASE.CO does not accept liability for your use of the website. The website is provided on an “as is” and “as available” basis, without any representations, warranties or conditions of any kind.
먹튀검증 ! 먹튀 피해를 예방하고 안전한 토토사이트 추천 리스트를 확인하세요. 먹튀검증소: https://mtverify.com/
토토사이트 ! 먹튀 피해를 예방하고 안전한 토토사이트 추천 리스트를 확인하세요. 먹튀감정사: https://www.danews.kr/
https://www.youtube.com/watch?v=RBMx7ZpUSXo
Simons Chase | Checkpoint Therapeutics Inc
anmpmxtcis
[url=http://www.g75k2lp49llci35b508tk6gllf29c371s.org/]unmpmxtcis[/url]
nmpmxtcis http://www.g75k2lp49llci35b508tk6gllf29c371s.org/
ブランドスーパーコピー
Cheap authentic items ugg ansley Birkenstock, Men's Mogami Terra Sandal – Black 'best prices online' under $130 exclusive shopping deals
FKM RUBBER SHEETING
Best offers for ugg bailey button sale Birkenstock, Men's Arizona EVA 2 Strap Sandal – Roast 'affordable shopping' under $140 online store deals
http://www.soonjung.net
china Automatic Winding Machine For Making Spiral Wound Gasket manufacture
Find authentic products cheap ugg store london SoftMoc, Women's Darilyn 2 Leather Chelsea Boot – Black Mauve 'online shopping' under $50 free shipping on orders
Where to shop online ugg fabrizia Birkenstock, Men's Arizona EVA 2 Strap Sandal – Elemental Blue 'price cuts' under $110 best e-commerce deals
Screw Extruder Machine
Pvc Extruders
Single Extruder
Medium Size Winder machine for making spiral wound gasket
Plastic Pellet Extruder
CHINA GLASS REINFORCED SILICONE SHEET
Single Extruder
Best deals on ugg adirondack boot ii Birkenstock, Men's Mogami Terra Sandal – Eucalyptus 'latest styles' under $120 trending online
china Medium Size Winder machine for making spiral wound gasket manufacture
Brown 3273 Phenolic Cotton Laminated Bar
Buy authentic for cheap ugg australia winter sale Skechers, Skechers, Boys' Guzman Steps Aqua Surge Sneaker – Black Red 'daily deals' under $50 online shopping discounts
Additives For Paints
Buy authentic items cheap ugg boots chestnut womens sale Skechers, Skechers, Infants' Guzman Steps AquaSurge Sneaker – Blue Lime 'huge discounts' under $170 best shopping offers
Where to buy discounted baby uggs Skechers, Skechers, Girls' Skechers Unicorn Backpack – Pink 'exclusive products' under $160 trending online
Phenolic cotton cloth laminated sheets
ブランドスーパーコピー
Solid Acid Catalyst
http://www.skarbek.fr.pl
Dehydrogenation Catalyst
Butyl Stannoic Acid
Carboxylic Acid Derivative
Diameter 16mm Phenolic Cotton Rod
High Temperature Resistance Bakelite Rod
Best discounts on cheap ugg boots Skechers, Skechers, Kids' 5 Piece Unicorn Backpack School Kit – Pink Multi 'price drop' under $130 online store promotions
Phenolic Cloth Laminate Rod 3025 10 Yarn
Online cheap shopping ugg boots pink sale 2024 Skechers, Skechers, Boys' Guzman Steps Aqua Surge Sneaker – Blue Lime 'popular brands' under $150 online exclusive sale
Wow amazing blog layout How long have you been blogging for you made blogging look easy The overall look of your web site is magnificent as well as the content
Get the best price uggaustralia Skechers, Men's Arch Fit Arcade Slip-Ins Sneaker – White 'top picks online' under $50 free shipping on orders
How to find best discounts ugg noira Skechers, Women's Uno Pop Back Lace Up Sneaker – White Coral 'deal of the week' under $50 best online bargains
Aluminum Oxide filled PTFE
Cheapest online deals uggboots Skechers, Men's Tres-Air Uno-Revolution-Airy Sneaker – Black White 'new arrivals sale' under $180 limited time discounts
ブランドスーパーコピー
Effluent Treatment Chemicals
Decolor Agent Water
Modified PTFE
Sludge Dewatering
vpxxi.ru
Nickel Filled PTFE
Polyacrylamide Flocculant
Glass Filled PTFE
Cheap Authentic childrens ugg boots Skechers, Women's Arch Fit Arcade Meet Ya There Sneaker – Black 'high demand' under $170 low price online
Buy cheap and original ugg slippers women Skechers, Women's Uno Pop Back Lace Up Sneaker – Black Hot Pink 'super savings' under $60 early bird deals
Papermaking Chemicals Industrial Lubricant
Ekonol Filled PTFE
I do not even know how I ended up here but I thought this post was great I do not know who you are but certainly youre going to a famous blogger if you are not already Cheers
of course like your website but you have to check the spelling on several of your posts A number of them are rife with spelling issues and I in finding it very troublesome to inform the reality on the other hand I will certainly come back again
Online shopping for authentic ugg slipper sale uk Heydude, Heydude, Baskets SIROCCO W NEUTRALS, crème, femmes 'shop today' under $90 buy today
Double Jacketed Gasket Machine
Corrugated Gasket with PTFE Coated
Silver Shower Set
http://www.pawilony.biz.pl
Reinforced Graphite Gasket
High Pressure Shower Head
Shower Set With Faucet
Graphite Gasket Reinforced with tanged metal
How to get cheap products ugg shoes sale uk Heydude, Heydude, Chaussure décontractée WENDY COMF SUEDE, noir, femmes 'huge discounts' under $100 best discount deals
Find authentic products cheap ugg logo UGG, Men's Classic Slip-On Sheepskin Slipper – Chestnut 'free shipping on orders' under $50 top trending products
Shop online for discounts ugg shop london UGG, Men's Biltmore Hiker Waterproof Boot – Stout 'hottest items' under $70 free shipping offers
Shower Faucet Set Black
Best place to shop online ugg manchester UGG, Women's Ashton Lace Up Boot – Black 'special offers' under $60 exclusive online discounts
Thermostatic Bath Filler Shower Set
ブランドスーパーコピー
Pure Graphite Gasket
RUBBER GASKETS AND SEALS
How to find cheap deals ugg cardy boots sale Skechers, Skechers, Baskets mode UNO STAND ON AIR, noir, femmes 'super savings' under $70 best prices guaranteed
Shoe Sole Injection Moulding Machine
How to find best discounts ugg cardy sweater boots Skechers, Skechers, Baskets mode UNO STAND ON AIR, blanc, femmes 'huge discounts' under $50 one day shipping
RX RING JOINT GASKET
Buy cheap and original ugg mini bailey button sale Skechers, Skechers, Baskets UNO STAND ON AIR, rosé, femmes 'deal of the week' under $60 quick delivery
Pu Sole Making Machine
plot28.com
Buy now ugg black sale boots Skechers, Skechers, Baskets UNO STAND ON AIR, noir, femmes – Large 'best prices online' under $150 order now
BX RING JOINT GASKET
Eva Sneaker Sole Making Machine
GRAPHITE GASKETS AND SEALS
ブランドスーパーコピー
SPIRAL WOUND GASKET
Machines For Making Shoes
Machine Making Rain Shoes
Cheap Authentic ugg classic short sale Skechers, Skechers, Baskets UNO STAND ON AIR, blanc, femmes – Large 'best value products' under $170 same day dispatch
Online best deals male ugg boots SoftMoc, Pantoufle Dini,châtain,mousse visco, fem 'shopping spree' under $100 online promotions
How to shop smart ugg fingerless gloves SoftMoc, Pantoufles en mousse visco. DINI, marine, femmes 'shop the sale' under $130 buy cheap online
Where to buy affordable kensington ugg boots sale SoftMoc, Botte Chelsea en cuir DARILYN 2, noir, femmes 'best online shopping' under $110 seasonal discounts
Colored Wire Sparklers
Artillery Shell Box
FLANGE ISOLATING GASKET KITS
pgusa.tmweb.ru
GLASS REINFORCED SILICONE SHEET
FKM FOOD QUALITY RUBBER SHEETING
Best shopping prices mens ugg SoftMoc, Botte en cuir DIANA LEATHER ZIP, noir, femmes 'affordable luxury' under $140 huge discounts
Assortement Package
Batterie
Buy affordable products leopard print ugg boots SoftMoc, SoftMocs DARIO, brun, hommes 'top picks online' under $120 fast and easy
Assortments Firework
FKM RUBBER SHEETING
HIGH TEMPERATURE SILICONE SHEETS
ブランドスーパーコピー
FKM FOOD QUALITY RUBBER SHEETING
CHINA FLANGE ISOLATING GASKET KITS
Buy authentic for less ugg accessories Puma, Baskets à lacets VIS2K, blanc rouge noir, hommes 'big offers' under $70 quick and fast shipping
Best online buys cheap ugg boots from china Puma, Baskets REBOUND FUTURE NEXTGEN, hommes 'holiday sale' under $90 trending e-commerce
Anti-Vibration Foaming
ブランドスーパーコピー
Phase Change Material
Insulating Curing Banding Tape
CHINA Small Size Winding Machine for Spiral Wound Gasket SUPPLIER
Shop for authentic kids ugg boots uk SoftMoc, SoftMocs avec fourrure de lapin CUTE 5, rouge, femmes 'popular items' under $50 special discounts
Silicon Foam
FLANGE ISOLATING GASKET KITS
How to buy cheap deals kids uggs uk Puma, Baskets à lacets CAVEN 2.0, noir blanc, hommes 'exclusive offers online' under $80 online exclusive discounts
fullsho.com
Shop for cheap deals ugg america Puma, Espadrille CALI DREAM, clou de girofle blc, femmes 'must-see' under $100 best price guarantee
Dog Bone
Small Size Winding Machine for Spiral Wound Gasket
How to buy cheap deals womens ugg boots SoftMoc, Women's Sadie Double Buckle Suede Sandal – Blue 'online promotions' under $80 trending products online
Refillable Perfume
Buy authentic for less girls ugg boots SoftMoc, Women's Double Buckle Crazyhorse Leather Sandal – Brown Crazyhorse 'great buys' under $70 special online sales
http://www.sceaindia.org
Rubber Sheet Reinforce with Cloth
ブランドスーパーコピー
H Shape Rubber Seal Strips
Online shopping for cheap ugg wedges SoftMoc, Women's Sadie Double Buckle Smooth Leather Sandal- Black 'new arrivals shopping' under $190 big deals
original solution
Black Natural Rubber Sheets
Natural Rubber Sheeting
Find affordable products ugg kensington boot SoftMoc, Women's Sadie Double Buckle Suede Sandal – Pink 'best markdowns' under $60 special offers
Where to buy discounted uggs for women SoftMoc, Men's Rollo Suede Plush Lined SoftMocs – Chestnut 'top product picks' under $160 order today
Empty Oil Bottles Wholesale
30ml Glass Spray Bottle
Natural Rubber Sheet
Oil Glass Bottle Dropper
Your writing is like a breath of fresh air in the often stale world of online content. Your unique perspective and engaging style set you apart from the crowd. Thank you for sharing your talents with us.
My brother suggested I might like this blog He was totally right This post actually made my day You can not imagine simply how much time I had spent for this info Thanks
MACHINE FOR SPIRALl WOUND GASKET RING AND STRIP
where to buy outfits 2024 Jordan Jordan Aerospace 720 UC sneakers Men female under $100 local boutiques
where to buy bloom outfits Jordan Air Jordan 33 future of flight Men for Girl under $100 eco-friendly clothing
Laser Machine
FULL AUTOMATIC KAMMPROFILE GROOVING GASKET MAKING MACHINE
ブランドスーパーコピー
where to buy ethereal Jordan Air Jordan 13 “Island Green” sneakers Men for guys under $100 quality apparel
http://www.lavidamata.xyz
FULL AUTOMATIC CAMPROFILE GROOVING GASKET MAKING MACHINE
Fiber Coupled Diode Laser Hair Removal Machine
BLACK CARBON FILLED PTFE TUBE
Anti-Freezing Membrane
Eswt Shockwave Therapy Machine
cheap Classy christmas outfits Jordan Air Jordan 5 Retro “Satin Bred” sneakers Men 2024 under $90 fashion sales
Laser Machine
cheap pumpkin patch outfits Jordan Air Jordan 13 Retro “Grey Toe” sneakers Men ideas male under $90 unique gifts near me
BRONZED FILLED WITH PTFE TUBE
RX RING JOINT GASKET
Fashion Belts
Suit Belt
Leather Belt Buckle
ブランドスーパーコピー
Nice Mens Belts
Buy authentic items cheap uggs boots on sale UGG, Men's Ascot Sheepskin Slipper – Chestnut 'on sale now' under $170 huge discounts online
GRAPHITE GASKETS AND SEALS
SPIRAL WOUND GASKET
Buy quality cheap products ugg tall boots DrMartens, Sandale à 2 brides VOSS, noir, femmes 'best offer' under $110 exclusive sales
Best online prices for ugg bailey button bomber DrMartens, Sandale BLAIRE SLIDE 3 STRAP, noir, femmes 'free returns' under $100 limited time discounts
How to get best deals tall uggs DrMartens, Sandale multibride BLAIRE, blanc, femmes 'price drop' under $70 top rated products
RUBBER GASKETS AND SEALS
BX RING JOINT GASKET
http://www.firesafety.ro
Buy products at low cost brown uggs DrMartens, Chelseas 2976 QUAD, blanc, femmes 'deal of the day' under $50 quick processing
Mens Elastic Belt
Ufo Led High Bay Light 200w
Online best deals bailey button ugg boots uk sale UGG, Botte de pluie Chelsea DROPLET, rouge samba, femme 'best bargains' under $100 best value
COMMERCIAL NEOPRENE INSERTION SHEETING – 1 PLY
Led Stadium Flood Lights
How to find discounts ugg shoe store UGG, Botte de pluie Chelsea DROPLET, taupe, femmes 'hot offers' under $90 next day delivery
NATURAL RUBBER SHEET
WHITE FOOD QUALITY RUBBER SHEETING
200w Ufo Led High Bay Light
FOOD GRADE NEOPRENE SHEETING – WHITE FDA
Cheap shopping options baby ugg slippers UGG, Botte d'hiver YOSE FLUFF V2, noir, femmes 'quick sale' under $80 customer reviews
gataquenha.com
1 PLY INSERTION RUBBER SHEET
Affordable online shopping ugg sandals UGG, Pantoufle COZETTA CURLY, noir, femmes 'popular shopping' under $70 featured products
ブランドスーパーコピー
Led High Bay Linear Light
200w Led Floodlight
Where to buy affordable ugg boots classic tall sale uk UGG, Botte de pluie Chelsea DROPLET, noir, femmes 'best price today' under $110 low price guarantee
Salt Water Filtration Systems
Oil-Resistance Asbestos Rubber Sheets
Non-Asbestos Jointing Sheets
Find affordable shopping brown ugg boots Birkenstock, Women's Buckley Casual Narrow Clog – Grey Taupe 'best price' under $180 fast and easy
Buy products online for cheap ugg hat Birkenstock, Women's Gizeh Braid Oiled Leather Thong Sandal – Black 'clearance sale' under $160 online promotions
Seawater Desalination Process Machinery
Where to find cheap products roslynn ugg boots Birkenstock, Women's Franca Buckle Sandal – White Patent 'discount offers' under $170 seasonal discounts
ブランドスーパーコピー
Ro Pure Water Making Machine
Tap Water Purifier Filter
http://www.okinogu.or.jp
Asbestos Rubber Sheets
Ro Seawater Desalination Machine
Find the best product deals ugg store uk Birkenstock, Women's Gizeh Big Buckle Thong Sandal – Black 'shop online' under $150 best value deals
Asbestos Rubber Sheet with wire net strengthening
Get the best online deals knitted ugg boots Birkenstock, Women's Franca Soft Footbed Buckle Sandal – Dove Grey 'exclusive offers' under $190 buy cheap online
Acid-Resistance Rubber Sheets