Leap Therapeutics Inc
LPTX is a clinical-stage immuno-oncology biotech. Their clinical programs primarily focus on inhibiting the Wnt signaling pathway and targeting Claudin18.2, both of which play crucial roles in cancer development and progression.
I met with management on December 13, 2023. The company’s $70 million +/- in cash is sufficient to reach upcoming readouts from two randomized clinical trials. The team is commercially-focused on getting to Phase 3 end points with business development options in place.
Baker Bros Advisors LP own nearly 20% of LPTX stock and warrants. Baker Bros first invested in LPTX in 2020 via Series A mandatorily convertible preferred stock. Series A converted into 14 million pre-funded warrants.
Wnt, also known as Wingless/Int-1, refers to a family of secreted glycoproteins that play crucial roles in various cellular processes, including embryonic development, cell fate determination, cellular proliferation, tissue morphogenesis, and stem cell function. They are involved in a diverse range of pathways, most notably the Wnt signaling pathway, which regulates a multitude of cellular activities.
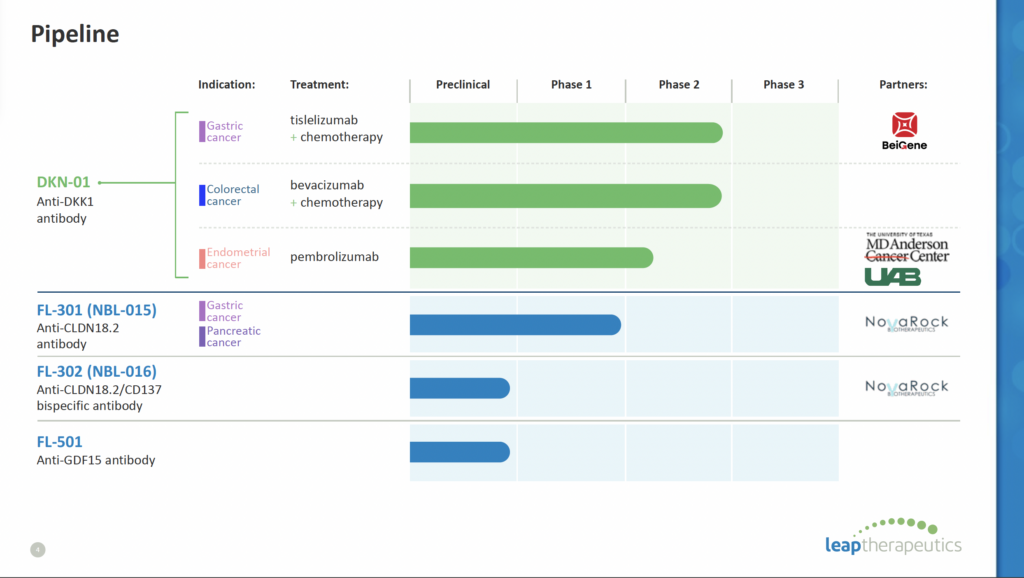
Wnt Signaling Pathway DKN-01 targets innate immunity by activating NK cells, reprogramming macrophages and inhibiting MDSCs, setting the stage for an enhanced adaptive immune response by anti-PD-1. This is a first-in-class DKK1 agonist currently being evaluated in multiple Phase 2 clinical trials across various cancer types, including:
- Esophagogastric cancer: DKN-01 is being investigated as a monotherapy and in combination with chemotherapy in Phase 2 trials.
- Gynecologic cancers: DKN-01 is being tested in combination with chemotherapy in Phase 2 trials for ovarian and endometrial cancers.
- Colorectal cancer: A Phase 2 trial (DeFianCe) is underway evaluating DKN-01 in combination with standard-of-care bevacizumab and chemotherapy for patients who have received prior systemic therapy. Part B uses mPFS as the primary endpoint and the benchmark varies from ~4.2m to 8.5m in this setting with Leap estimating ~6m as the target mPFS.
- FL-501: This is a humanized monoclonal antibody targeting Claudin18.2, currently in Phase 2 development for patients with gastric and pancreatic cancer.
Claudin18.2 FL-301: This is another humanized monoclonal antibody targeting Claudin18.2, currently in Phase 1/2 development for patients with advanced solid tumors. Preclinical Programs: LPTX also has preclinical programs targeting other novel targets, including:
- 2/CD137: This program combines the Claudin18.2 targeting of FL-301 with the immunostimulatory activity of CD137 to potentially enhance anti-tumor immune response.
- GDF15: This program focuses on developing therapeutic antibodies against GDF15, a protein associated with tumor growth and metastasis.
Key Partnerships:
- MD Anderson Cancer Center: Collaboration involves research and clinical development of DKN-01 for pancreatic cancer.
- Memorial Sloan Kettering Cancer Center: Collaboration focuses on the development of FL-301 for gastric cancer.
- The Wistar Institute: Collaboration involves research on the role of Claudin18.2 in cancer and the development of FL-301.
- BeiGene
LPTX plans to Advance DKN-01 through Phase 2 clinical trials and into pivotal Phase 3 trials, continue developing FL-301 and FL-501 in Phase 2 clinical trials and potentially expand into additional cancer indications. Also, the company will further explore the potential of its preclinical programs, such as Claudin18.2/CD137 and GDF15.
Investors are advised to conduct their own independent research into individual stocks before making a purchase decision. In addition, investors are advised that past stock performance is not indicative of future price action.
You should be aware of the risks involved in stock investing, and you use the material contained herein at your own risk. Neither SIMONSCHASE.CO nor any of its contributors are responsible for any errors or omissions which may have occurred. The analysis, ratings, and/or recommendations made on this site do not provide, imply, or otherwise constitute a guarantee of performance.
SIMONSCHASE.CO posts may contain financial reports and economic analysis that embody a unique view of trends and opportunities. Accuracy and completeness cannot be guaranteed. Investors should be aware of the risks involved in stock investments and the possibility of financial loss. It should not be assumed that future results will be profitable or will equal past performance, real, indicated or implied.
The material on this website are provided for information purpose only. SIMONSCHASE.CO does not accept liability for your use of the website. The website is provided on an “as is” and “as available” basis, without any representations, warranties or conditions of any kind.
먹튀검증 완료 안전한 메이저 토토사이트 리스트를 확인하세요. 다양한 이벤트와 혜택도 함께 제공됩니다. https://mtverify.com/
먹튀검증 완료 안전한 메이저 토토사이트 리스트를 확인하세요. https://mtverify.com/
“Such a refreshing read! 💯 Your thorough approach and expert insights have made this topic so much clearer. Thank you for putting together such a comprehensive guide.”
Grow and ovarian enlargement priligy usa
Your blog is a testament to your dedication to your craft. Your commitment to excellence is evident in every aspect of your writing. Thank you for being such a positive influence in the online community.
Fantastic site Lots of helpful information here I am sending it to some friends ans additionally sharing in delicious And of course thanks for your effort
helloI really like your writing so a lot share we keep up a correspondence extra approximately your post on AOL I need an expert in this house to unravel my problem May be that is you Taking a look ahead to see you
Your writing has a way of resonating with me on a deep level. It’s clear that you put a lot of thought and effort into each piece, and it certainly doesn’t go unnoticed.
Wow wonderful blog layout How long have you been blogging for you make blogging look easy The overall look of your site is great as well as the content
obviously like your website but you need to test the spelling on quite a few of your posts Several of them are rife with spelling problems and I to find it very troublesome to inform the reality on the other hand Ill certainly come back again
Your blog is a testament to your dedication to your craft. Your commitment to excellence is evident in every aspect of your writing. Thank you for being such a positive influence in the online community.
먹튀검증커뮤니티: https://offhd.com/
Simply wish to say your article is as amazing The clearness in your post is just nice and i could assume youre an expert on this subject Well with your permission let me to grab your feed to keep updated with forthcoming post Thanks a million and please carry on the gratifying work
Hey there You have done a fantastic job I will certainly digg it and personally recommend to my friends Im confident theyll be benefited from this site
Every time I visit your website, I’m greeted with thought-provoking content and impeccable writing. You truly have a gift for articulating complex ideas in a clear and engaging manner.
Somebody essentially help to make significantly articles Id state This is the first time I frequented your web page and up to now I surprised with the research you made to make this actual post incredible Fantastic job
Somebody essentially lend a hand to make significantly posts I might state That is the very first time I frequented your web page and up to now I surprised with the research you made to create this particular put up amazing Excellent job
Somebody essentially lend a hand to make significantly articles I’d state. That is the very first time I frequented your website page and up to now? I surprised with the research you made to make this actual submit amazing. Wonderful task!